Abstract
Thrombotic microangiopathies (TMA) constitute a group of life-threatening diseases of different aetiologies characterized by similar symptoms. The compilation of comprehensive data related to the initial clinical presentation of TMA is challenging due to their rare occurrence.
The objective of this study was to assess and determine the predictive value of the clinical presentation of patients with a suspected TMA episode, subsequently confirmed as thrombotic thrombocytopenic purpura (TTP), compared with non-TTP TMA by taking advantage of the centralized ADAMTS-13 testing in Quebec.
All ADAMTS-13 activity and antibody titration were performed at CHU Saint-Justine (CHUSJ). Measurements and patient demographics (from April 2012 to December 2019) were extracted from the Laboratory Information System of the CHUSJ. Patients with presumed TMA were classified into TTP when ADAMTS-13 activity was ≤10%, or suspicion of TMA other than TTP (non-TTP TMA) when ADAMTS-13 activity was >10%. Clinical presentation was obtained through a survey form received with each plasma sample and containing information related to prior episodes of TMA, underlying conditions (pregnancy, cancer, infection, transplant, medication, other), clinical symptoms (fever, neurological signs, abdominal signs), and biological parameters (hemolytic anemia, thrombocytopenia). Statistical analyses were performed with IBM SPSS 26.0. The study was approved by the Research Ethics Committee of CHUSJ.
A total of 2081 requests for ADAMTS13 testing were received during the study period, in 846 different individuals with suspected TMA: 147 patients (17%) had a confirmed TTP and 699 had a suspected non-TTP TMA. Clinical and biological characteristics associated with TMA suspicion were available for 105 TTP and 470 non-TTP TMA patients (68% of subjects).
More than half of patients with TTP (55%; 48/87) had neurological signs at presentation compared to one third of patients (33%; 124/375) with non-TTP TMA (p=0.0001). There were no significant differences regarding fever and abdominal signs between the 2 groups (p=0.383 and p=0.355 respectively). Anemia and thrombocytopenia were reported in 92% (80/87) and 93% (86/92) of TTP patients compared to 74% (291/396) and 83% (352/426) of non-TTP TMA patients (p=0.0002 and p=0.009, respectively). Underlying conditions were reported in 62% (287/460) of non TTP-TMA patients compared to 35% (34/97) of TTP patients (p<0.0001). Kidney involvement was documented in 20% (91/460) of non-TTP TMA and 7% (7/98) of TTP (p=0.003). Transplantation tended to be more often reported in non-TTP TMA (11%;58/560 versus 4%;4/98 in TTP; p=0.079). Pregnancy or postpartum was found in 11% (29/267) of females with non-TTP TMA and 5% (3/57) of females with TTP (p=0.232). Infections were present in 15% (68/462) of non-TTP TMA and 14% (14/98) of TTP (p=0.912). Drug associated TMA was reported in 15% (70/461) of non-TTP TMA and 9 % (9/98) of TTP (p=0.123). Cancers were documented in 16% (74/462) of non-TTP TMA and 9% (9/98) of TTP (p=0.084).
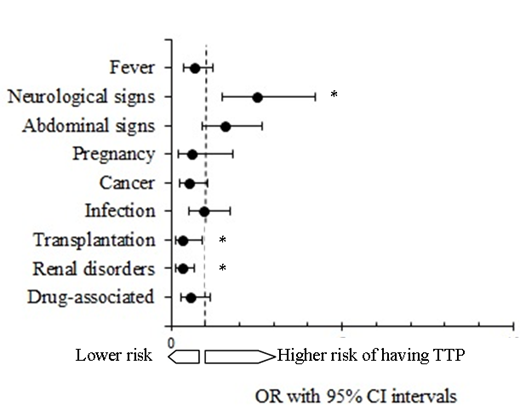
When addressing the odds of TPP according to the clinical presentation, patients with neurological signs were at higher risk to be diagnosed with TTP compared to those who did not have neurological signs (odds ratio, 2.52; IC95%, 1.51 to 4.20; p<0.001). Thirty percent of patients presenting with neurological signs had TTP compared to 14% presenting without neurological signs. Conversely, the risk to be diagnosed with TTP was lower in the setting of transplantation (odds ratio, 0.32; IC95%, 0.11 to 0.92; p=0.015). Indeed, among patients who have had a transplantation 7% were diagnosed with TTP, while 93% had non-TTP TMA. Finally, the risk of TPP was also lower in patients with TMA and concomitant kidney-related issue (OR=0.31; IC95%: 0.14 to 0.69; p=0.004) as among patients presenting with renal disorders, 93% were not subsequently diagnosed with TTP.
In conclusion, the centralization of ADAMTS-13 activity testing in one reference center has enabled to determine the predictive value of clinical characteristics associated with TPP in comparison to other types of TMA for the entire province of Quebec, Canada. This study provides useful insight for caregivers and paves the way to the establishment of a provincial registry of TMA patients.
Lapeyraque: Alexion Pharmaceuticals Inc: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Rivard: Bayer Inc: Membership on an entity's Board of Directors or advisory committees, Research Funding; Octapharma Inc: Membership on an entity's Board of Directors or advisory committees; Pfizer Inc: Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk Inc: Membership on an entity's Board of Directors or advisory committees. Bonnefoy: Alexion Pharmaceuticals Inc: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi Genzyme Inc: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring Inc: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.